Much of what we do is related to the preparation of compounds with defined stereochemistry. This can involve asymmetric synthesis, the use of enantiomerically-pure starting materials or resolutions. Stereochemistry is particularly important when dealing with biologically active materials such as pharmaceuticals and agrochemicals where apparently small changes can have major effects.
Organometallic reagents play a major role in our research. Organometallic reagents are particularly useful in organic synthesis since they can be used to make new carbon-carbon bonds, often with stereochemical consequences. We have used organic derivatives of aluminum, boron, copper, lithium, magnesium, tin, and zinc to effect asymmetric transformations. While a wide range of metals is used, the underlying theme is the same: to make new carbon-carbon bonds with control of stereochemistry. Some specific examples are noted below.
Aminostannanes have tremendous potential as building blocks for the synthesis of biologically important compounds such as β-amino alcohols and α-amino acids. We have explored the chemistry of α-aminostannanes and developed protecting groups which can be used in the transformations shown below. However, applications of this chemistry are limited by efficient routes to enantiomerically-pure α-aminostannanes.
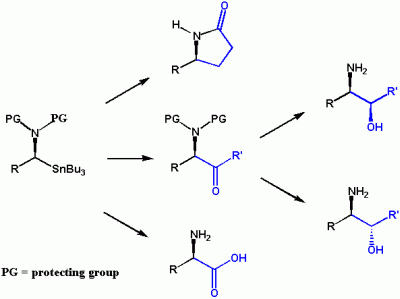 |
We have recently developed an efficient and highly stereoselective route to homochiral a-aminostannanes. We have shown that certain α-aminostannanes can be used in palladium-catalyzed (Stille) couplings. Such couplings will permit access to a wide range of amines which are not easily prepared by other methods. We are particularly interested in the stereochemistry of these couplings. Applications to the synthesis of pharmaceutically-important materials such as β-blockers will also be examined.
Binaphthol (below left, Y = H) has been an extremely useful ligand for asymmetric synthesis. During the course of our work on developing an asymmetric alkynylating reagent, we found that boronates (below right, Z = alkynyl) could efficiently transfer alkynyl groups to enones with very high (up to >99:1) enantioselectivities. We also developed new methods to modify binaphthols sterically and electronically to control reactivity patterns and selectivities.
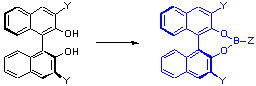
We have applied these boronates to the allylation of aldehydes and ketones and discovered some very high selectivities. We are actively examining modification of these reagents to homochiral Lewis acid catalysts or other reagents to open new pathways for new methodology. A particularly exciting discovery is that certain reactions can be run with only catalytic amounts of binaphthols to afford products with high stereochemical purity.
The use of organomagnesium reagents to effect carbon-carbon bond formation has been a cornerstone of synthetic organic chemistry since they were introduced by Victor Grignard over a century ago. Over the past few decades, many attempts have been made to chirally modify Grignard reagents to produce reagents that can enantioselectively alkylate carbonyl compounds. While some successes have been reported, the relatively high intrinsic reactivity of Grignard reagents towards aldehydes and ketones has made it difficult to achieve high stereoselectivities. We have recently found that dialkylmagnesiums react with chiral amines to produce chiral organomagnesium amides (COMAs) which appear to be more reactive than the parent dialkylmagnesiums. This discovery has far-reaching ramifications in terms of developing highly selective systems which may require only a catalytic amount of chiral ligand. Thus far, we have shown that COMAs can alkylate aldehydes and reduce trifluoromethyl ketones with high selectivities. We have obtained the first X-ray crystal structure of a COMA (see below). This structure will help us to design other chiral ligands which may give even higher selectivities.
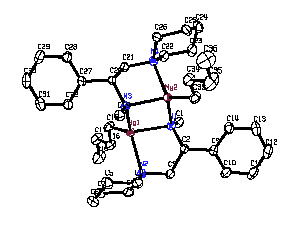 |
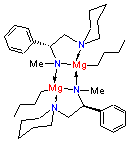 |
Currently, we are exploring other asymmetric transformations for which COMAs may be useful. We are also preparing new ligands which may be useful in these reactions or other asymmetric processes. As well, the development of catalytic systems (using magnesium as well as other metals) is underway.
Insect pheromones are an important component of integrated pest management (IPM) programs. They are gaining commercial importance as a "natural" or "green" alternative to pesticides since their biological activity is usually species-specific. Pheromones are attractive targets for us since they are usually fairly simple molecules where stereochemistry can have profound effects on activity. The classic example of such effects is the sex pheromone of the gypsy moth, (+)-disparlure where <1% of the other enantiomer can significantly decrease potency. We have worked with government agencies such as the Canadian Forestry Service (CFS) and the United States Department of Agriculture (USDA) to develop effective syntheses of various pheromones. Work is continuing in this area to develop better syntheses of old pheromones and also to design syntheses of new pheromones. Pheromones that we have made include:
In pursuing any of the above projects, students will gain experience in the skills expected of a synthetic organic chemist such as inert atmosphere (Schlenk line, syringe, glove box) techniques, purification methods (flash chromatography, distillation, recrystallization), and analytical techniques (HPLC, GC, GC/MS, IR, NMR). In addition, and perhaps more importantly, students are expected to think critically and develop into independent researchers.